Chlorierung von Latex Kleidung
Wir haben Latex Kleidung schon bereits vor 20 Jahren auf Kundenwunsch chloriert und sehr viel Erfahrung in diesem Bereich gesammelt.
Seit kurzer Zeit bieten wir bei einigen Kleidungsstücken wo es möglich ist auch eine einseitige Chlorierung an. Diese vereint die Vorteile von chlorierten und unbehandelten Latex. Lesen Sie dazu mehr weiter unten.
Immer mehr Kunden fragen uns, welche Vor- und Nachteile mit einer Chlorierung von Latex Kleidung einhergehen und ob ein Latex Catsuit oder andere Latex Kleidungsstücke auch eigenständig chloriert werden kann. Oftmals findet man im Internet Anleitungen zur Selbstchlorierung, jedoch sind diese oftmals nicht in der nötigen Tiefe chemisch korrekt und tragen zum kursierenden Halbwissen über den Chlorierungsprozess negativ bei.
Aus den genannten Gründen möchten wir Ihnen die Vorzüge und Nachteile der Chlorierung von Latex Kleidung detailliert erläutern und eine brauchbare, korrekte Anleitung zum Chlorieren zur Verfügung stellen. Um den Prozess des Chlorierens besser verstehen zu können, ist es zunächst sinnvoll sich zumindest vereinfacht mit der Herstellung und Chemie des Latex zu befassen. Die historisch-wissenschaftlich weniger interessieren Leser können die entsprechenden Abschnitte natürlich überspringen…
1. Latex - vom Baum zum chlorierten Latex Catsuit
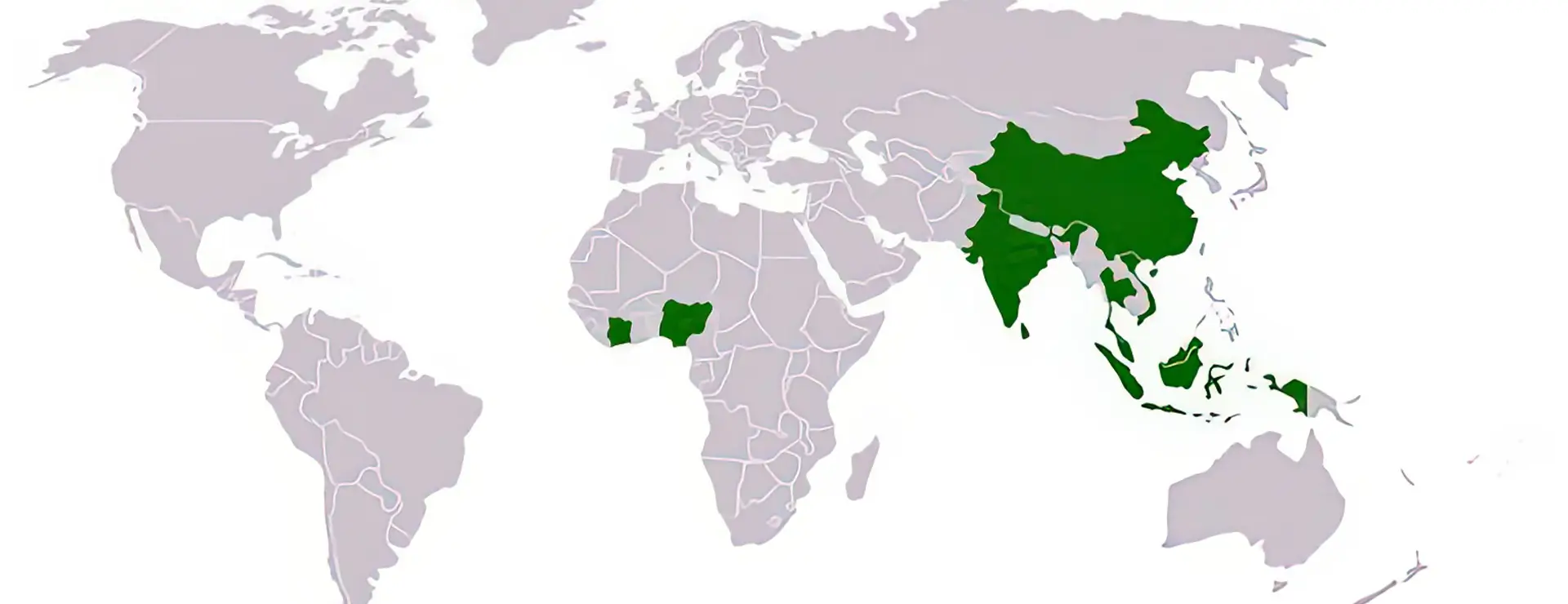
Latex, genauer gesagt Gummi, ist ein Naturprodukt und legt einen langen Weg bis in Ihren Kleiderschrank zurück. Auf großen Plantagen in Malaysia, Indien oder Brasilien werden Kautschukbäume (hevea brasiliensis) angebaut und ihre Rinde mit einer speziellen Technik eingeritzt um an den weißen Milchsaft des Kautschukbaumes zu gelangen. Diese sogenannte Latexmilch enthält bis zu 20% natürlichen Kautschuk. Zur Extraktion des Kautschuks (indian. cao ‚Baum‘ und ochu ‚Träne‘; zusammen „Träne des Baumes“) aus der Latexmilch wird diese zunächst eingedickt und der Kautschuk durch Zugabe verdünnter Essigsäure ausgefällt und anschließend getrocknet. Die Jahresproduktion beläuft sich auf etwa 7.6 Millionen Tonnen und verteilt sich nach Rang geordnet auf insgesamt 17 Produktionsländer.
| Rang | Land | Produktion (in Tsd. Tonnen) |
|---|---|---|
| 1 | Thailand | 3030 |
| 2 | Indonesien | 1792 |
| 3 | Malaysia | 1000 |
| 4 | Indien | 694 |
| 5 | China | 550 |
| 6 | Vietnam | 391 |
| 7 | Elfenbeinküste | 123 |
| 8 | Nigeria | 112 |
| 9 | Liberia | 108 |
| 10 | Brasilien | 96 |
| 11 | Sri Lanka | 92 |
| 12 | Philippinen | 88 |
| 13 | Guatemala | 50 |
| 14 | Kambodscha | 46 |
| 15 | Kamerun | 46 |
| 16 | Myanmar | 36 |
| 17 | Mexico | 23 |
Der so erhaltene gelblich gefärbte Naturkautschuk weißt jedoch noch keine Werkstoffeigenschaften auf. Es handelt sich um eine extrem viskose Masse, welche bei längerer Lagerung unter ihrem Eigengewicht langsam „zerfließt“. Diese Eigenschaft wird als „Visko-Elastizität“ bezeichnet. Latexkleidung und unzählige technische Anwendungen des Gummis wurden erst durch die von Charles Goodyear im Jahr 1839 entdeckte Vulkanisation ermöglicht. Kautschuk besteht auf molekularer Ebene aus sehr langen Kohlenwasserstoff-Ketten. Diese sind gegeneinander frei beweglich und sorgen dadurch für die zähflüssige Konsistenz. Bei der Vulkanisation wird der Kautschuk zusammen mit Schwefel und weiteren Additiven unter Druck erhitzt. Der Schwefel vernetzt die Ketten untereinander und sorgt auf diese Weise für zusätzliche Stabilität und insbesondere für die Elastizität des resultierenden Gummis (Latex). Über den Schwefelgehalt können dabei die Materialeigenschaften präzise gesteuert werden - je mehr Schwefel, desto härter und unelastischer wird das erhaltene Latex. Das Zustandekommen der Werkstoffeigenschaften ist am einfachsten in Analogie zu einer Sprossenleiter zu verstehen: Der rohe Naturkautschuk entspricht dabei den losen Holmen der Leiter - ohne Sprossen sind sie beliebig gegeneinander beweglich. Bei der Vulkanisation wirkt der Schwefel wie die Sprossen zur Verbindung der Holme - je mehr Sprossen zwischen den Holmen eingebaut werden, desto stabiler wird die Leiter. Zur Herstellung von Latexkleidung wird nur sehr wenig Schwefel eingesetzt. Das Ergebnis ist eine „wackelige“ Leiter mit gegeneinander verschiebbaren Holmen bzw. elastisches Latex (Gummi) für Ihre Lieblingskleidung. Das Latex wird hierbei direkt in Bahnen hergestellt und kann anschließend durch einen Klebstoff miteinander verbunden werden, welcher den Vulkanisationsprozess nachahmt und die Latexschichten sicher miteinander verbindet. Auf diese Weise ist es uns möglich für Sie Kleidung auch auf Maß anzufertigen - auf den Zentimeter genau. Wer perfekte Latex Kleidung zudem besonders schnell anziehen möchte, sollte sich mit der Chlorierung des Materials beschäftigen. Im Folgenden möchten wir Ihnen alles Wissenswerte zum Prozess der Chlorierung vermitteln sowie eine Anleitung zur Chlorierung im Selbstversuch bereitstellen.
2. Chlorierte Latex Kleidung - Vorteile und Nachteile
Durch das wunderbare Tragegefühl ist man natürlich schnell dazu verleitet, seinen geliebten Latexanzug zu chlorieren, doch die Behandlung mit Chlor weist nicht nur Vorteile auf. Beispielsweise verändert sich die Oberfläche des Latex derart, dass Verklebungen beinahe unmöglich werden und somit Reparaturen stark erschwert bis unmöglich sind. An dieser Stelle sei gesagt dass wir seit kurzem (bei Modellen wo es möglich ist) eine einseitige Chlorierung anbieten.
Vorteile:

Schnelles Anziehen ohne Hilfsmittel
Durch die Chlorierung wird die Oberfläche des Latex glatt und seidig. Das Gummi gleitet auf der Haut und kann ohne Hilfsmittel wie Silikonöl oder Talkum angezogen werden. Daher ist chlorierte Latex Kleidung besonders bei großen und aufwendig anzuziehenden Kleidungsstücken (z.B. Latex Catsuit) empfehlenswert. Der gleitende Effekt geht jedoch verloren, wenn die Haut feucht ist (Schwitzen) oder zuvor eingecremt wurde.

Kein Verkleben der Latex Kleidung
Die Latex Kleidung verklebt weder im nassen noch im trockenen Zustand. Dies spart Nerven und erleichtert die Handhabung. Es ist möglich die Kleidung im Trockner bei Kaltluft zusammen mit einem weichen Handtuch aufzupolieren. Es entsteht ein Grundglanz. Eine Tiefenpflege, z.B. mit Silikonöl, ist jedoch dennoch anzuraten.

Latex Kleidung für Allergiker geeignet
Chloriertes Latex kann so aufbereitet werden, dass es für Allergiker geeignet oder zumindest besser geeignet ist. Dies begründet sich mit der Zerstörung des allergenen Proteins, welches im Latex eingeschlossen ist. Dazu sollte die Latex Kleidung aber vorher gründlich (mind 24 Stunden) gewässert werden.

Höhere Haltbarkeit
Unbehandeltes Latex weist innerhalb des Kohlenstoff-Rückgrats des Materials sogenannte Doppelbindungen auf. Diese stellen „Andockstellen“ für Radikale dar, welche das Latex nachhaltig schädigen können und die Langlebigkeit verringern. Durch das Chlorieren werden diese Doppelbindungen zu etwa 70% zerstört und die chemische Beständigkeit des Latex deutlich erhöht.
Nachteile:

Für manche Kleidungsstücke ist Chlorierung nur bedingt geeignet
Größere Kleidungsstücke, welche wenig Tendenz zum „Rutschen“ haben sind sicherlich Favoriten wie z.B. Latex Catsuits, Hemden oder Latex Mäntel. Jedoch Strümpfe, Miniröcke, kurze Latex Kleider können durch die besonders glatte Oberfläche des chlorierten Latex leicht herunterrutschen (Tipp: Den Rand von der Leggings etwa 10 cm umschlagen und mit etwas Wasser befeuchten. Nach dem Zurückschlagen wirkt das Wasser als Haftvermittler und die Leggings rutscht nicht mehr). Natürlich kann diese Methode auch bei anderen Kleidungsstücken angewandt werden. Normale (Baumwoll-)kleidung über chloriertem Latex rutscht ebenfalls stark. Lieber den Gürtel bedenken, wenn man nicht im Freien stehen mag.

Reparatur erschwert/unmöglich
Der größte Nachteil: Nur dicke Latex Kleidung kann ansatzweise durch die „Schleifmethode“ zufriedenstellend repariert werden.
Der olfaktorische Reiz schwindet
Geruchs-Enthusiasten werden es schwer haben. Der ursprüngliche Geruch weicht vollständig und wird durch einen neuen ersetzt. Besonders wenige Tage nach der Chlorierung ist noch ein restlicher Chlorgeruch wahrnehmbar - dieser verschwindet jedoch vollständig. Der neue Geruch kann als „industrieller“ beschrieben werden. Bei dickerem Latex werden die innersten Schichten des Latex wenig bis gar nicht chloriert. Hier ist es möglich, dass ein gewisser Restgeruch bis nach Außen vordringt. Für die Masse der chlorierten Kleidung sollte man sich jedoch auf den veränderten Geruch einstellen.
Veränderung der Haptik
Das Latex wird fester und steifer. Anfangs ist dieser Effekt deutlich zu spüren - durch längeres Tragen, wird das Material wieder etwas weicher. Durch die veränderte Haptik wirkt das chlorierte Latex subjektiv dicker als vergleichbares, unbehandeltes Gummi. Ein dünner Latex Catsuit aus 0.25 mm Latex fühlt sich z.B an, als sei er aus 0.35 mm Latex gefertigt worden.
Komplette Chlorierung: Alle Aspekte im Überblick
| Vorteile | Nachteile |
|---|---|
| Anziehen ohne Hilfsmittel | Komplikationen bei manchen Kleidungsstücken (z.B Strümpfe) |
| Kleidung verklebt nicht | Reparatur erschwert / unmöglich |
| Für Allergiker geeignet | Veränderter Geruch |
| Höhere Haltbarkeit / Beständigkeit | Veränderte Haptik |
| Pflegeleichter als unchloriertes Latex |
NEU - einseitige Chlorierung (SSC) - Alle Aspekte im Überblick
| Vorteile | Nachteile |
|---|---|
| Anziehen ohne Hilfsmittel | Anfangs etwas veränderter Geruch (verschwindet mit der Zeit) |
| Kleidung verklebt nicht | Aufwendiger und daher etwas teurer |
| Für Allergiker geeignet | |
| Höhere Haltbarkeit / Beständigkeit | |
| Pflegeleichter als unchloriertes Latex | |
| Reparatur sichtbar von außen möglich | |
| Besonders angenehme Griffigkeit |
3. Unsichtbare Änderungen - trage ich noch Latex?
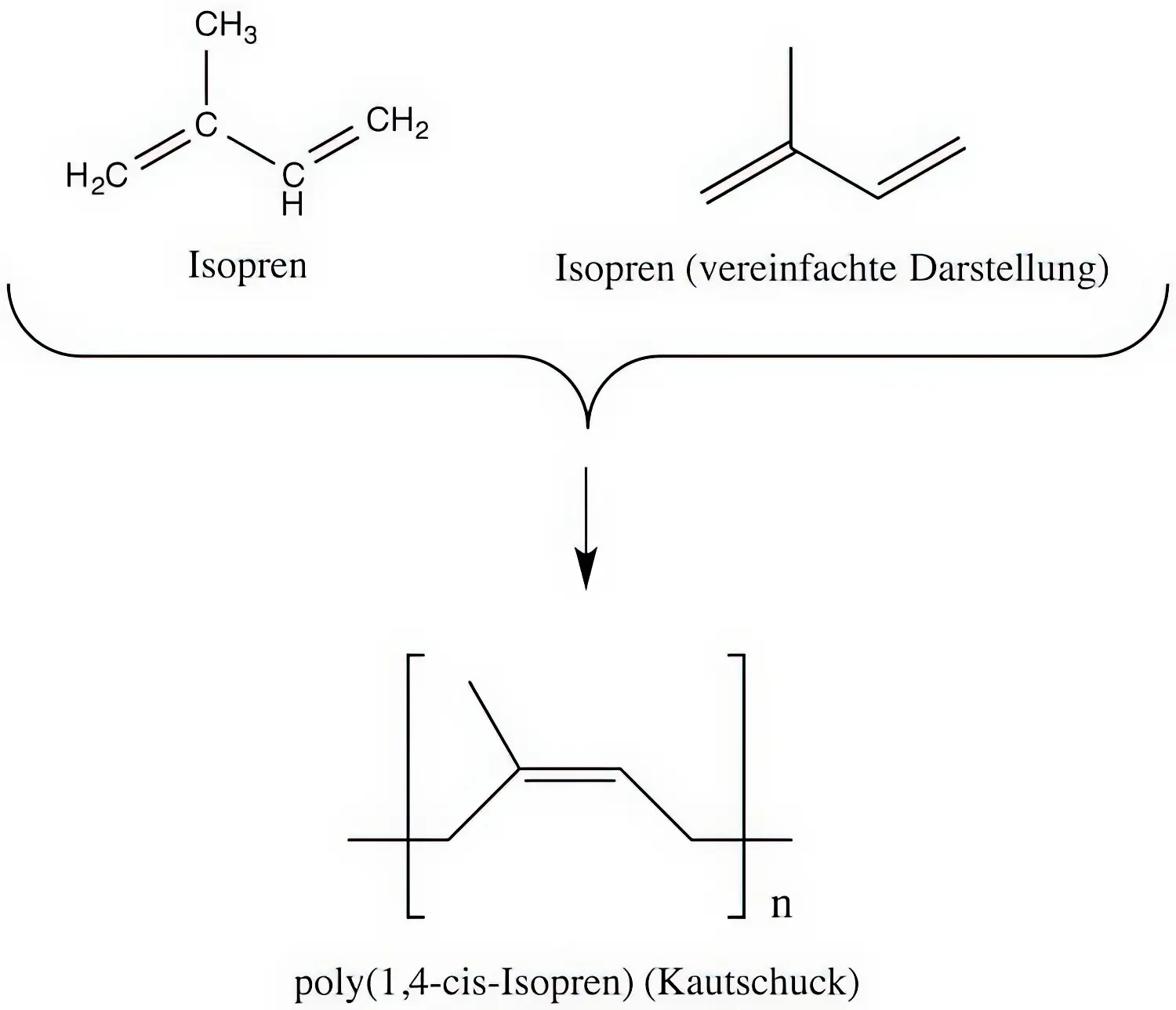
Der nachfolgende Abschnitt ist eher für die Freizeit-Chemiker oder besonders interessierten Leser gedacht. Um die Frage der Überschrift vernünftig klären zu können müssen wir Latex kurz auf chemischer, also molekularer Ebene betrachten. Kautschuk ist ein Bio-Polymer, welches aus sogenannten Isopren-Monomeren (Grundbaustein) aufgebaut ist. Im Kautschuk sind dabei etwa 30.000 Isopren-Moleküle zu langen Ketten verknüpft. Die Verknüpfung wird dabei mit einem kleinen Index „n“ symbolisiert (n = 30.000).
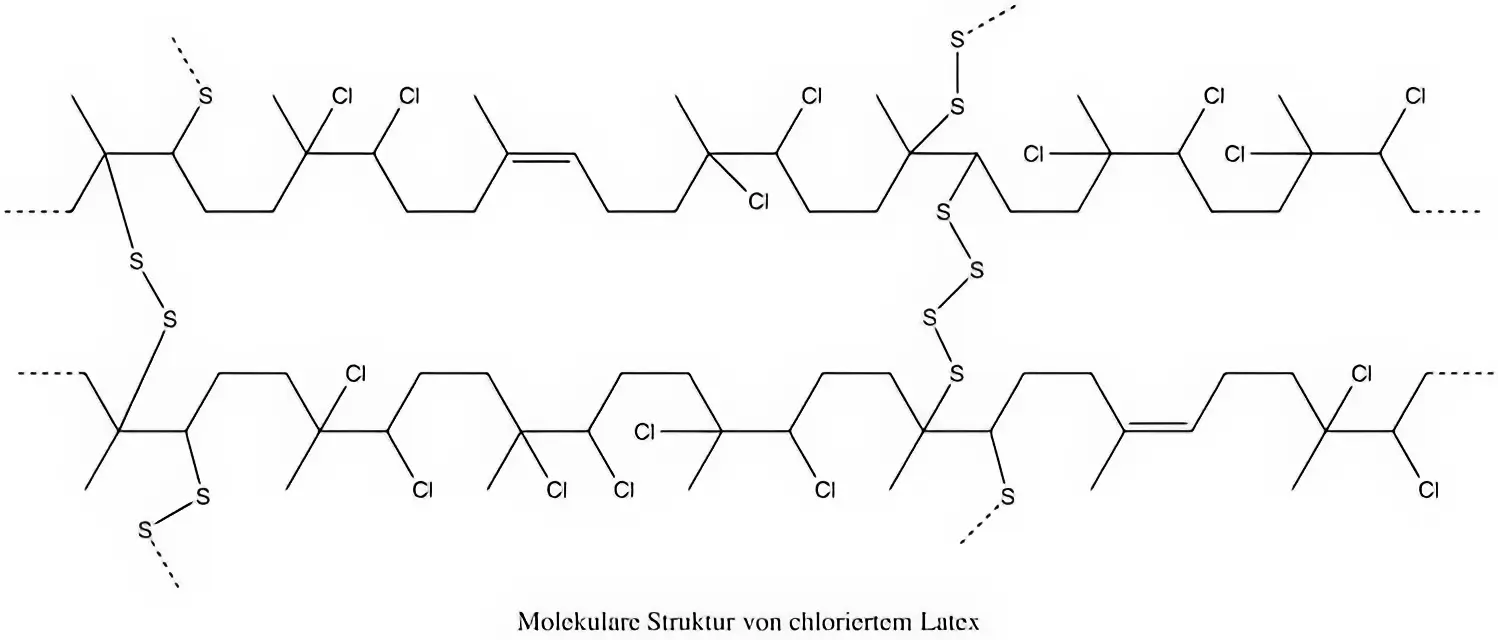
Was ist eine Softchlorierung ?
Manche Hersteller bieten eine Softchlorierung an. Eine wirkliche Softchlorierung gibt es eigentlich nicht - es handelt sich dabei lediglich um eine nicht vollständig Chlorierung deren Ergebnis oft nicht zufriedenstellend ist und keinen Vergleich mit einer richtigen Chlorierung darstellt. Eine Chlorierung ist erst dann korrekt und vollständig durchgeführt, wenn alle reaktiven Bindungsstellen im Material mit Chlor „gesättigt“ wurden. Manchmal klebt die Latex Kleidung trotzdem noch und eine Anziehhilfe ist notwendig. Darüber hinaus tendiert das soft-chlorierte Latex dazu das Chlor bei Sonneneinstrahlung oder erhöhten Temperaturen wieder abzuspalten. Dieses Prozess ist sehr langsam und nicht gefährlich aber allemal ärgerlich.
4. Anleitung zum Chlorieren & Sicherheitshinweise
Nun zum eigentlichen Kerngeschäft- der Chlorierung selbst. Hierfür werden einige Chemikalien benötigt, von welchen bei unsachgemäßer Handhabung diverse Gefahren ausgehen können. Aus diesem Grund ist es ratsam die folgende Anleitung exakt zu befolgen und die gegebenen Sicherheitshinweise sorgfältig zu berücksichtigen.
Benötigte Materialien
- Salzsäure (30%)
- Natriumhypochlorit-Lösung (14%)
- Natriumcarbonat (Soda)
- 2x Kunststoffeimer (PE, PP oder PTFE)
- Chemikalienschutzhandschuhe (Butyl-Kautschuk)
- Schutzbrille
- Gasmaske mit Aktivkohlefilter
- Eventuell alte Kleidung oder Schürze/Kittel
Sicherheitshinweise:
Anmerkung zu den Sicherheitsdatenblättern: Die Datenblätter beinhalten Beschreibungen der Stoffe und deren Gefahrenpotential. Die Angaben beziehen sich hierbei jedoch auf industrielle Tonnenmaßstäbe. Bei der Latexchlorierung arbeiten wir mit Kleinstmengen und bei genauer Einhaltung der Vorschrift und Verwendung der angeführten Schutzkleidung ist die Chlorierung als unbedenklich anzusehen. Die Sicherheitsdatenblätter werden der Information und Vollständigkeit halber zur Verfügung gestellt.
Durchführung:
Aufgrund der Giftigkeit und ätzenden Wirkung des entstehenden Chlors ist der Versuch in Räumen ausschließlich bei guter Durchlüftung und angelegtem Atemschutz (Gasmaske) durchzuführen!
Reinigen Sie zuerst das Latex von etwaigem Silikonöl, Schmutz oder Kleberresten. Für ein gutes Ergebnis sollte der Anzug gründlich mit einem milden Waschmittel gereinigt werden. Befüllen Sie nun beide Eimer mit je 5 L Wasser. Der erste Eimer dient der Chlorierung, der zweite zum Abwaschen der Reaktionsmischung nach dem Chlorieren. Füllen Sie mit einem Messbecher 120 mL Natriumhypochlorit-Lösung (14%ig) ab und geben Sie die Lösung in Eimer 1. Geben Sie den Latex Catsuit nun in den Eimer und walgen ihn in der Lösung, damit er gleichmäßig mit der Natriumhypochlorit-Lösung in Kontakt kommt. Füllen Sie nun 20 mL Salzsäure (30%ig) ab und geben Sie die Säure in den Eimer 1. Hierbei kann es kurz dampfen (nicht erschrecken). Sobald die Salzsäure zugegeben wurde, bildet sich Chlorgas, welches mit dem Latex zu reagieren beginnt.
NICHT EINATMEN! LEBENSGEFAHR!
Walgen Sie das Latex nun für 3 Minuten gleichmäßig in der Reaktionslösung und wenden Sie es häufig. In dieser Zeit wird der größte Teil des Chlors mit dem Latex abreagieren. Um ein optimales Ergebnis zu erzielen, belassen Sie das Kleidungsstück noch weitere 1-2 Minuten in der Mischung, wobei Sie das Walgen des Latex unterlassen können, da eine gleichmäßige Grundchlorierung bereits gegeben ist und nur vereinzelt noch Chlor in das Latex eingebaut wird. Entnehmen Sie nun den chlorierten Latex Catsuit und geben Sie ihn in den Eimer 2. Wenden Sie ihn dabei mehrfach und walgen Sie den Catsuit dabei im klaren Wasser. Hierdurch wird die Reaktionsmischung und überschüssiges Chlor zum größten Teil entfernt. Entnehmen Sie den Catsuit aus Eimer 2 und waschen Sie ihn z.B in einem Waschbecken noch 3x gründlich aus. Wechseln Sie nach jedem Spülgang das Wasser. Nun ist Ihre Kleidung sicher von überschüssigem Chlor befreit. Beim Waschen beigefügtes Spülmittel kann helfen, den Chlorgeruch schneller zu beseitigen. Hängen Sie Ihren Latex Catsuit nun wie gewohnt zum Trocknen auf. Nun muss noch die Reaktionsmischung in Eimer 1 entsorgt werden. Geben Sie dazu mit einem Esslöffel Natriumcarbonat zu der Mischung bis kein Zischen bzw. Aufschäumen der Mischung mehr beobachtet werden kann. Ist dies der Fall, ist die Lösung pH-neutral und kann problemlos über den Ausguss entsorgt werden. Aufgrund der Verdünnung können Sie das Wasser aus Eimer 2 ebenfalls einfach in den Ausguss entsorgen.
Latex Chlorierung für Allergiker:
Unter bestimmten Voraussetzungen ist chloriertes Latex für Allergiker geeignet. Dazu muss die Latex Kleidung 24 h in kaltem Wasser vollständig untergetaucht werden und anschließend mit frischen Wasser ausgewaschen werden. Diese Prozedur vermindert den Gehalt an allgergenem Protein im Latex. Durch die folgende Chlorierung wird das Protein beinahe vollständig zerstört. Durch den verminderten Allgergen-Gehalt ist das chlorierte Latex auch für Allergiker tragbar. Dieses Verfahren ist für medizinische Latexartikel bereits etabliert.
Tipps & Ratgeber zum Thema Latex und mehr
Simon O. Magazin

Behind the Scenes: Simon O. Latex-Fotoshooting neuer Modelle

Die Ursachen von Latex Allergien und wie man sie reduzieren kann.